Accelerate Study Execution
Launch complex protocols with advanced workflows and data capture for faster data availability.
Boost productivity with intuitive processes, automated EMR capture, and integrated performance dashboards.
Accelerating Research For
Embleema unites investigators, site teams, and patients to advance precision medicine. Our platform combines site-based and decentralized features to accelerate study implementation, streamline data capture, return personalized patient data, and deliver compliant real-world evidence.
Backed by 100+ years of leadership and proven partnerships with the FDA, DoD, and leading life sciences organizations, Embleema helps research teams launch studies faster, adapt quickly, and achieve transformative results. Known for our technology and collaborative approach, we deliver exceptional value to sponsors and researchers.
Combined experience in life sciences, healthcare data, and blockchain.
Advancing research across the US, Europe, and Asia.
Work with and trust Embleema to drive innovation.

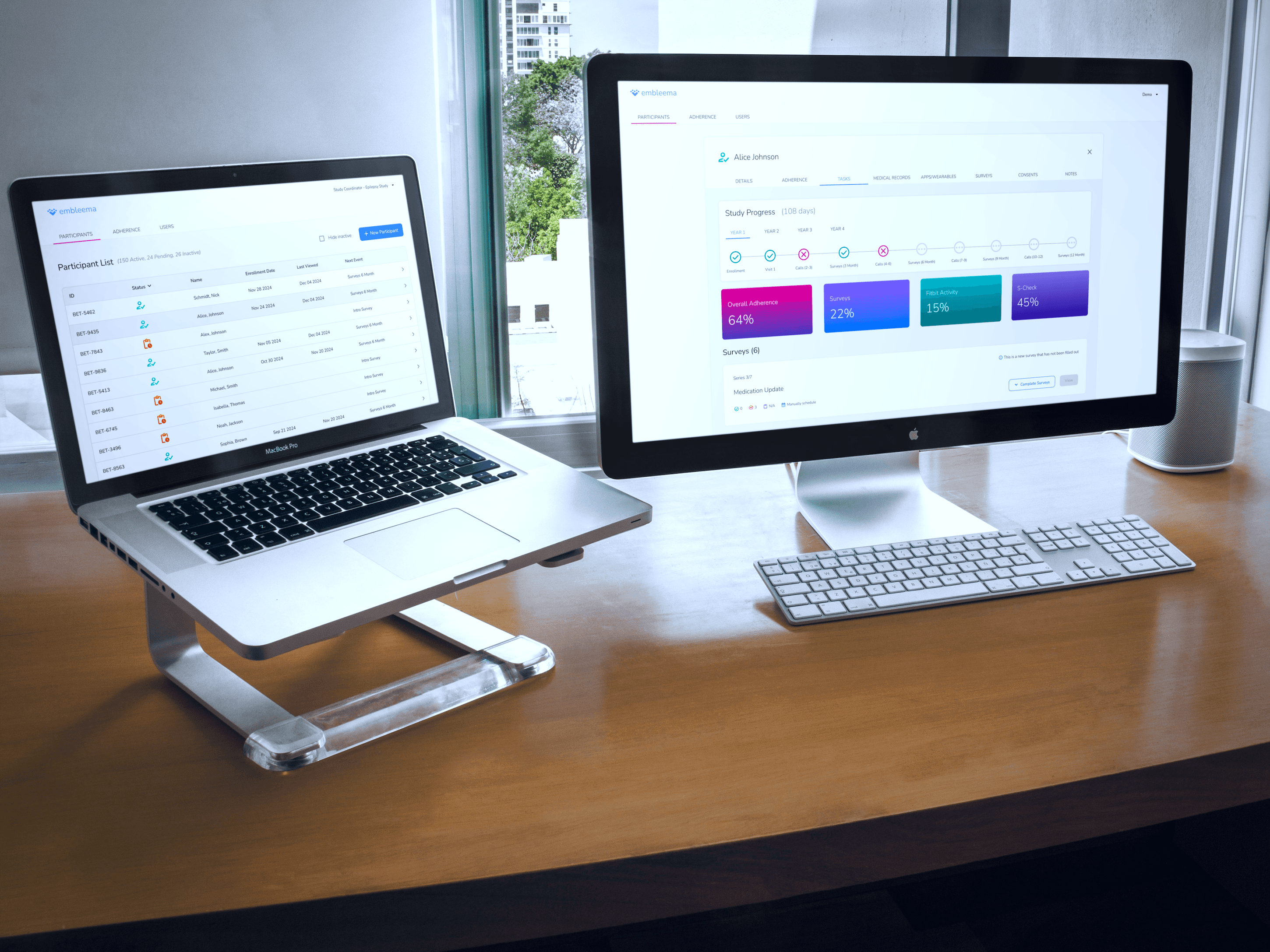
All-in-One Research Platform
Our specialists quickly configure your observational studies or registries in our flexible, no-code platform, handling complex protocols, consolidating multi-modal data, and adapting to evolving needs—all within a single portal.
Simplify execution with smart workflows, automated scheduling, and centralized data streams
Integrate EHR, imaging, molecular, biospecimen, and patient data for richer insights
Access real-time enrollment and performance dashboards
FOR clinical Sites
Advanced Site Tools
Attract and retain top KOL investigators while maximizing site efficiency through intelligent automation of data collection and study management.
Unify data streams into a single platform for streamlined access
Reduce errors and accelerate timelines with EMR integration and intelligent prefill
Maintain high-quality, compliant data with built-in oversight features
for patients
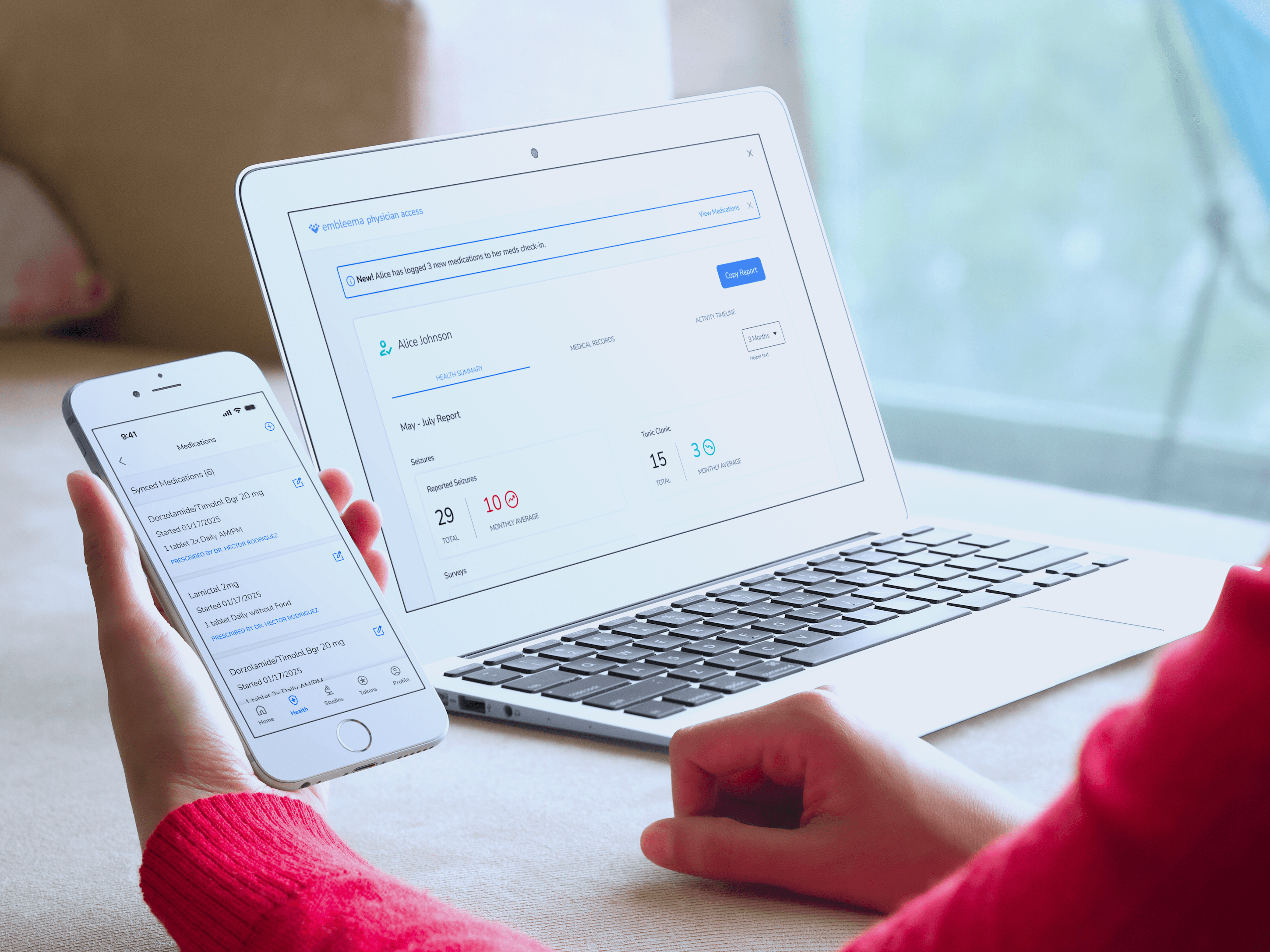
Participant Engagement App
Offer participants a modern app that collects critical study data while returning valuable health insights—making them true partners in discovery. Available on web, iOS and Android.
Keep participants engaged with easy check-ins and real-time feedback
Enable secure access to medical records and remote e-consent
Provide health metrics, updates, and insights to sustain engagement
33%
more study tasks completed vs. other methods
97%
completion rate for eCRFs and CLINROs in 2024 for sites using Embleema
60,000
care centers connected
— Jacqueline French, MD, Neurology Professor and Epilepsy Study Consortium President
















